MES for Pharmaceutical Manufacturing
Cloud-Based MES | Electronic Batch Manufacturing Records | 21 CFR Part 11 Compliance
Pharmaceutical manufacturers face continuous pressure to remain competitive while meeting stringent regulatory requirements. NGIMES helps you comply with 21 CFR Part 11, FDA audits, validation, and cGMP requirements—all on a cloud-based platform.
Schedule Your DemoWhy NGIMES for Pharma Manufacturing?
Proven experience delivering on every aspect of regulated pharmaceutical manufacturing with accelerated performance
Complete Regulatory Compliance
Built-in compliance with 21 CFR Part 11 electronic signatures, FDA audit readiness, validation, and cGMP requirements. Everything you need for regulatory confidence.
- FDA 21 CFR Part 11 signatures
- cGMP enforcement at every step
- Complete audit trail
Electronic Batch Records
Complete eBMR data capture without compromising data integrity. Analyze and understand root causes of out-of-specification products with complete traceability.
- Paperless batch execution
- Real-time data capture
- Root cause analysis tools
Cloud-Based Excellence
Run on SAP HANA platform with in-memory computing for accelerated performance. Contain costs while delivering enterprise-level capabilities.
- SAP HANA in-memory performance
- Reduced infrastructure costs
- SAP worldwide support
NGIMES: Comprehensive Manufacturing Coverage
End-to-end manufacturing execution from quality to production to compliance
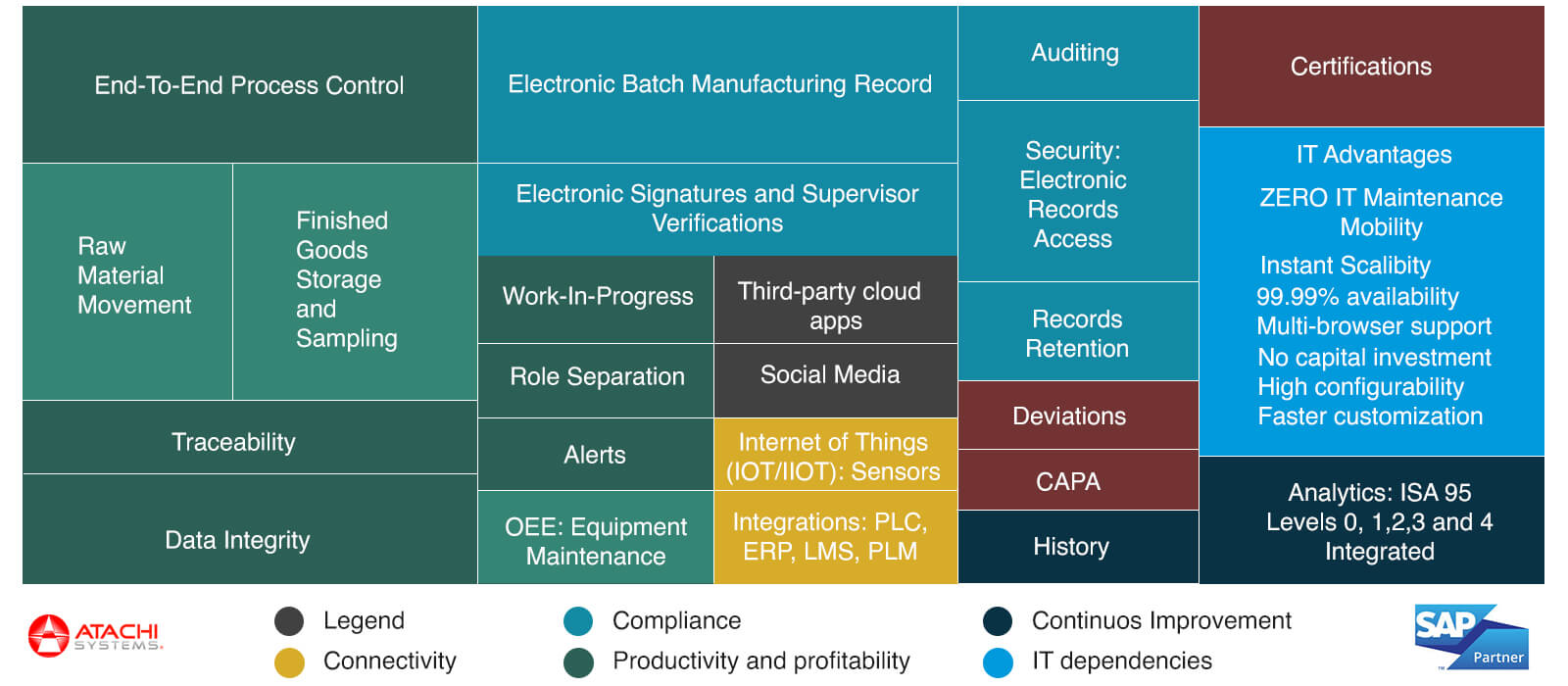
Complete Manufacturing Capabilities
Everything you need for pharmaceutical manufacturing excellence
cGMP Compliance
Manufacturing Execution Systems enforcing strict conformity to cGMP requirements to ensure that right procedures are used at every manufacturing step.
Continuous Improvement
Corrective and Preventive actions (CAPA) identified, documented and implemented for ongoing process optimization and quality enhancement.
API Stability for Active Pharmaceutical Ingredient Manufacturers
API manufacturers face critical challenges proving API stability through the stability period. Our system provides a simplistic process that avoids API stability issues and reduces FDA audit response times to as low as an hour.

Why API Stability Matters
Recent FDA warning letters to API manufacturers highlight the importance of proving API stability with complete data integrity. NGIMES provides the documentation structure and data integrity needed to avoid FDA compliance issues.
Data Integrity: Your Competitive Advantage
Data integrity issues are putting pharmaceutical manufacturers out of the US consumer market. Don't let it be a growth killer for your business.
Common MES Challenges
- Too expensive to own and maintain
- Too difficult to hire and retain talent to manage MES
- Too difficult to put MES projects on right track and realize ROI
- Too difficult to do seamless integration across all systems
NGIMES Solution
With cloud technology and SAP HANA, the rules have been rewritten. NGIMES makes MES affordable and accessible for small and mid-size pharmaceutical industries.
- Cloud-based reduces ownership costs
- SAP worldwide support reduces talent needs
- Proven implementations ensure ROI
- Native SAP integration built-in

Proven Experience in Pharmaceutical Manufacturing
Atachi Systems has the experience and knowledge to deliver on every aspect of regulated pharmaceutical manufacturing. Our hands-on experience working with various MES systems in the marketplace is unmatched.
Young and fast-growing Indian pharmaceutical companies have shown keen interest in migrating to NGIMES because of its solid performance on SAP HANA and unique industry-specific pricing structure. Combined with SAP worldwide support and training, NGIMES is the obvious choice for leading-edge companies.
Ready to Transform Your Pharma Manufacturing?
Learn how NGIMES can help you achieve FDA compliance, data integrity, and operational excellence
Talk To Us Today About Your MES Needs